


| Cambridge study identifies astrocyte network as new therapeutic target for Parkinson’s disease |  Preview Preview
 download download |
| Eli Lilly acquires biotech ‘newcomer’ Versanis for $2bn |  Preview Preview
 download download |
| The 10th Central and Western Forum of China Cancer Rehabilitation Organization was held — the national cancer rehabilitation was established at the meeting |  Preview Preview
 download download |
| Digital Pregnancy Rapid Test |  Preview Preview
 download download |
| Digital Ovulation Rapid Test |  Preview Preview
 download download |
| Pregnancy Rapid Test |  Preview Preview
 download download |
| Semi-quantitative Ovulation Rapid Test |  Preview Preview
 download download |
| Ovulation Rapid Test |  Preview Preview
 download download |
| DC908A Multi-Drug Test Cup Reader |  Preview Preview
 download download |
| Marijuana (THC) Saliva Test Midstream |  Preview Preview
 download download |
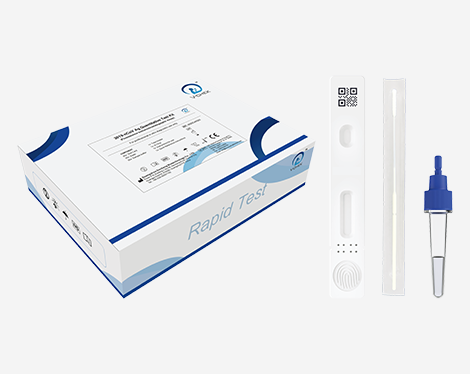

This kit is used for in vitro quantitative detection of 2019-nCoV antigen. This Kit is a lateral flow immunofluorescent sandwich assay that is used with the immunofluorescence analyzer intended for the quantitative detection of the nucleocapsid protein antigen from 2019-nCoV in nasopharyngeal (NP) swab specimens directly or after the swabs have been added to viral transport media from individuals who are suspected of COVID-19 by their healthcare provider.
This test is only for clinical laboratory use or for immediate inspection by medical personnel, not for home testing, and cannot be used as the basis for the diagnosis and exclusion of pneumonia caused by new coronavirus infection.
A positive test result needs further confirmation. A negative test result cannot rule out the possibility of infection.
The kit and test results are for clinical reference only. It is recommended to combine the patient’s clinical manifestations and other laboratory tests for a comprehensive analysis of the condition.
This Kit is intended for use by medical professionals or trained operators who are proficient in performing tests using an immunofluorescence analyzer.
STORAGE AND STABILITY
Related product
